The electromagnetic spectrum has its origins in the study of electromagnetism and the behavior of electromagnetic waves. It emerged as a result of the work of several physicists and scientists over the 19th and 20th centuries who sought to understand and describe the nature of electromagnetic radiation.
Thank you for reading this post, don't forget to subscribe!Table of Contents
Introduction of Electromagnetic Spectrum
One key origin point for the concept of the electromagnetic spectrum is the development of James Clerk Maxwell’s equations in the mid-19th century. Maxwell’s equations mathematically unified the understanding of electricity and magnetism and predicted the existence of electromagnetic waves, including light. This groundbreaking theoretical work laid the foundation for our modern understanding of the electromagnetic spectrum.
An Analogy with Rainbow:
One analogy that helps illustrate the concept of the electromagnetic spectrum is the idea of a “rainbow.” A rainbow is a visible spectrum of colors that occurs when sunlight is refracted, or bent, as it passes through water droplets in the atmosphere. Each color in the rainbow represents a different wavelength of visible light.
The electromagnetic spectrum is a continuum of all the different types of electromagnetic radiation, arranged by their wavelengths and frequencies. It encompasses a wide range of energy, from the lowest-energy radio waves used for communication to the high-energy gamma rays associated with nuclear processes.
Electromagnetic Spectrum Significance in Physics:
In physics, the electromagnetic spectrum plays a fundamental role in understanding the behavior of electromagnetic waves and their interaction with matter. It is a cornerstone of the study of electromagnetism, helping physicists explain phenomena such as the behavior of light, electricity, and magnetism. Maxwell’s equations, a set of fundamental equations in electromagnetism, describe how electromagnetic waves propagate through space and how they interact with charged particles.
Significance in Everyday Life:
The electromagnetic spectrum has a profound impact on our daily lives, even though we may not always realize it. Here are some ways in which it is significant in everyday life:
Communication:
Radio waves and microwaves are used for wireless communication, including radio broadcasts, television signals, cell phone calls, and Wi-Fi connections.
Healthcare:
X-rays and gamma rays are essential for medical imaging, allowing doctors to diagnose conditions and monitor treatment.
Heating and Cooking:
Microwaves are used in microwave ovens to heat and cook food quickly and efficiently.
Vision:
Visible light, a part of the spectrum, enables us to see and perceive the colors and details of our surroundings.
Remote Sensing:
Infrared and ultraviolet rays are used in remote sensing technologies, such as weather forecasting and satellite imagery.
Energy Production:
Solar panels capture sunlight (visible and some infrared radiation) and convert it into electricity.
Security:
Infrared sensors are used in security systems and motion detectors.
Physicists and Scientists who made significant contributions
The study and understanding of the electromagnetic spectrum have been the result of the contributions of numerous physicists and scientists over the years. Here are listed some notable :
James Clerk Maxwell:
In 1865, Maxwell formulated the basic laws of electromagnetism in four equations, are fundamental to our understanding of electromagnetism and the behavior of electromagnetic waves. They provided a theoretical framework that unified electricity and magnetism and predicted the existence of electromagnetic waves, including speed of the light. Later, after his death Hertz generated and detected electromagnetic waves experimentally.
Heinrich Hertz:
Hertz conducted experiments in the late 19th century that confirmed the existence of electromagnetic waves and demonstrated that they could be generated and detected. His work provided experimental validation of Maxwell’s equations.
Albert Einstein:
Einstein’s work on the photoelectric effect, for which he received the Nobel Prize in Physics in 1921, contributed to the understanding of how electromagnetic radiation (in the form of photons) interacts with matter.
Max Planck:
Planck’s work on blackbody radiation and the quantization of energy led to the development of quantum mechanics, which has deep implications for our understanding of the electromagnetic spectrum at the atomic and subatomic levels.
Niels Bohr:
Bohr’s atomic model, which incorporated quantized electron energy levels, contributed to the understanding of how atoms interact with electromagnetic radiation, particularly in the context of atomic spectra.
Visible Light Spectrum and EM Wavelengths
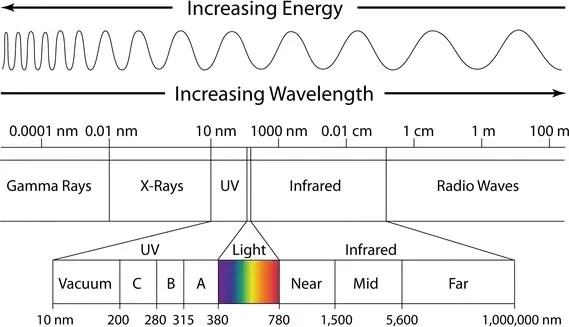
Later on X-rays and Gamma-rays were discovered, which shows electromagnetic behavior and travels with the speed of light. The wavelength of these rays were much larger than the visible light, so all these are put in an order. This order of wavelengths for different electromagnetic waves is called electromagnetic spectrum. It includes visible light, radio waves, microwaves, infrared, ultraviolet, X-rays, and gamma rays. A simple diagram to illustrate the spectrum’s divisions and wavelengths is given below.
Properties of Different Regions in EM Spectrum:
Lets break down each region of the electromagnetic spectrum, focusing on their key properties, examples, and numerical values. We will start from the region with the longest wavelengths and lowest frequencies and move towards the region with the shortest wavelengths and highest frequencies:
1. Radio Waves:
Wavelength: Radio waves have the longest wavelengths, ranging from about 1 millimeter to hundreds of meters.
Frequency: They have the lowest frequencies, typically in the range of 3 kHz (kilohertz) to 300 GHz (gigahertz).
Key Properties: Radio waves are primarily used for communication. They can travel long distances and penetrate buildings and obstacles. They are employed in AM/FM radio broadcasting, television signals, cell phone calls, and radar systems.
Value: AM radio broadcasts typically use frequencies around 1 MHz (megahertz), while FM radio operates in the range of 88 MHz to 108 MHz.
Uses: Radio and TV communications.
2. Microwaves (Short Radio Waves):
Wavelength: Microwaves have shorter wavelengths, typically ranging from 1 millimeter to 1 meter.
Frequency: Frequencies for microwaves fall in the microwave oven, and satellite communication ranges, which can be from 1 GHz to 300 GHz.
Key Properties: Microwaves are used in microwave ovens for cooking food by exciting water molecules. They are also essential for satellite communication, Wi-Fi networks, and radar technology.
Value: Wi-Fi operates around 2.4 GHz and 5 GHz, while microwave ovens use a frequency of about 2.45 GHz.
Uses: Long distance wireless communications via satellites, microwave ovens and radars.
3. Infrared (IR):
Wavelength: Infrared radiation has even shorter wavelengths, typically ranging from 1 millimeter down to 700 nanometers (nm).
Frequency: Frequencies for IR radiation are in the terahertz (THz) range.
Key Properties: Infrared radiation is associated with heat. It’s used in infrared thermometers for temperature measurement, remote controls, and night vision technology.
Value: Infrared thermometers operate in the range of about 8 μm (micrometers) to 14 μm.
Uses: In green houses, to look through fog or mist.
4. Visible Light:
Wavelength: Visible light is the narrow band of electromagnetic radiation that humans can perceive, with wavelengths ranging from approximately 380 nm (violet) to 750 nm (red).
Frequency: Frequencies for visible light are in the hundreds of THz.
Key Properties: Visible light is responsible for human vision and the perception of colors. It’s used in photography, displays, and optical communication.
Value: The wavelength of green light is around 550 nm.
Uses: Electrons presence in different states of the atoms (from radiation)
5. Ultraviolet (UV):
Wavelength: Ultraviolet radiation has shorter wavelengths than visible light, typically ranging from about 10 nm to 380 nm.
Frequency: Frequencies for UV radiation are in the hundreds of THz and petahertz (PHz).
Key Properties: UV light has germicidal properties and is used in sterilization processes. It’s also responsible for sun tanning and sunburn. UV lamps are used in various applications, including counterfeit detection.
Value: The wavelength of UVA (long-wave UV) is around 320 nm, while UVB (medium-wave UV) is around 280 nm.
Uses: Detection of forged documents, fingerprints, and to see invisible writing.
6. X-rays:
Wavelength: X-rays have very short wavelengths, ranging from about 10 picometers (pm) to 10 nanometers (nm).
Frequency: Frequencies for X-rays are in the exahertz (EHz) range.
Key Properties: X-rays are used in medical imaging (X-ray radiography and CT scans) to visualize the inside of the body, as well as in airport security scanners.
Value: Medical X-rays typically use energies ranging from 20 keV (kiloelectronvolts) to 100 keV.
Uses: In medical, and to study structure of the atoms and crystals.
7. Gamma Rays:
Wavelength: Gamma rays have the shortest wavelengths, less than 10 picometers (pm).
Frequency: Frequencies for gamma rays are in the zettahertz (ZHz) range.
Key Properties: Gamma rays are extremely high-energy and are produced during nuclear reactions. They are used in nuclear medicine for cancer treatment and imaging.
Value: The energy of gamma rays can range from hundreds of keV to several MeV (megaelectronvolts).
Uses: It provide structure information of the nuclei.
8. Long Waves:
Wavelength: The corresponding wavelength range for long waves can vary, but it generally spans from meters to hundreds of kilometers.
Frequency: Long waves typically have frequencies that range from a few kilohertz (kHz) to hundreds of gigahertz (GHz).
Lower Frequency Limit: Around 3 kHz (kilohertz)
Upper Frequency Limit: Up to several hundreds of GHz (gigahertz)
Corresponding Wavelength Range: From approximately 100 meters to several kilometers
Here’s the table of electromagnetic spectrum with frequency ranges expressed using powers of 10 (scientific notation) for simplicity:
| Region | Frequency Range (Hz) | Wavelength Range (meters) |
|---|---|---|
| Radio Waves | 3 x 103 Hz – 3 x 1011 Hz | 1 x 102 meters – 1 x 10-3 meters (1 millimeter) |
| Microwaves | 3 x 108 Hz – 3 x 1011 Hz | 1 x 10-3 meters (1 millimeter) – 1 meter |
| Infrared (IR) | 3 x 1011 Hz – 4.3 x 1014 Hz | 7 x 10-7 meters (700 nanometers) – 1 x 10-3 meters (1 millimeter) |
| Visible Light | 4.3 x 1014 Hz – 7.5 x 1014 Hz | 3.8 x 10-7 meters (380 nanometers) – 7.5 x 10-7 meters (750 nanometers) |
| Ultraviolet (UV) | 7.5 x 1014 Hz – 3 x 1016 Hz | 1 x 10-8 meters (10 nanometers) – 3.8 x 10-7 meters (380 nanometers) |
| X-rays | 3 x 1016 Hz – 3 x 1018 Hz | 1 x 10-10 meters (10 picometers) – 1 x 10-8 meters (10 nanometers) |
| Gamma Rays | 3 x 1018 Hz and higher | Less than 1 x 10-10 meters (less than 10 picometers) |
NOTE:
1 picometer = 10-12 meters.
Hz (Hertz) is the standard unit and represents one cycle per second.
KHz (Kilohertz) = (103 Hz)
MHz (Megahertz) = (106 Hz)
GHz (Gegahertz) = (109 Hz)
THz (Terahertz) = (1012 Hz)
PHz (Petahertz) = (1015 Hz)
EHz (Exahertz) = (1018 Hz)
ZHz (Zettahertz)= (1021 Hz)
Numerical problem electromagnetic spectrum:
1Q. Compute photon energy (in eV) in gamma rays and visible regions of electromagnetic spectrum . Relate these energies to the corresponding sources of electromagnetic radiation. Given: h=6.63×10-34 Js, c= 3.0×108 m/s and 1eV = 1.6 × 10-19J.
Solution:
We have two photons one of gamma rays and second of visible spectrum(may be of any color). We have to show their energies, and have to understand the source of radiation.
So first point here is about to the energy of a photon and formula for that. I hope you will be aware about this;
E= hν -(1)
h is the Planck’s constant that is given and v is the frequency, that can be define in terms of the wavelength (λ) also,
ν = c/λ;
So, here λ is unknown, that value you have to put, as per the table 1., given above ; so I choose λ = 10-10 meter for gamma rays and 3.8 x 10-7 meter for visible light.
Also, you have to keep in your mind the units of these constants and parameters. So, when you will put the value in the equation 1, its unit will be in Joule. So to convert it in eV you have to conversion unit given above in the question i.e., 1eV = 1.6 × 10-19J.
So now for the gamma ray’s photon the energy will be;
E = hν = h c/λ
E = (6.63 × 10-34 Js) × (3.0 × 108 m/s) / 10-10 meter
E = 19.89 × 10-16 Joule
E = 19.89 × 10-16 / 1.6 × 10-19 eV
E= 12.43 × 103 eV
or E= 12.43 keV
If you will take wavelength 10-12 meter then E= 1.243 MeV, so energy is dependent on the wavelength.
Now, for the visible light;
E = (6.63 × 10-34 Js) × (3.0 × 108 m/s) /(3.8 × 10-7 meter)
E = 5.23 × 10-19 J
E = 5.23 × 10-19 /1.6 × 10-19 eV
E = 3.27 eV for the visible light.
So now you can understand the difference of the energy for the photons emitted through gamma rays and in visible spectrum.
Few questions that can be answered based on the above discussion.
Which prove that electromagnetic waves are transverse in nature?
Is the nature of electromagnetic wave transverse stationary?
What is the transverse nature of EM waves formula?
Why are electromagnetic waves always transverse?
Are all electromagnetic waves transverse?
What is the wavelength of a transverse wave *?
What is the nature of electromagnetic waves?
What is called transverse wave?
What is an example of a transverse wave?
How are transverse waves measured?
What is the speed of transverse wave?
Do transverse waves have speed?
What is transverse size?
What is the formula of the transverse wave speed?
What is the equation for a transverse magnetic wave?
What is the equation for the transverse electric mode?
What is the formula of wave nature of light?
What is transverse wave in physics?
How do you find a transverse wave?
What is transverse magnetic?
What is the SI unit of a transverse wave?
What are the 3 equations for wave speed?
What is the formula for longitudinal and transverse waves?
If you want to know more please let me know.